Reflection 10- Nucleotides and Nucleic Acids
Hey y’all! It’s the one and only Shiv Shiv here again. Well it’s not been so long since I blogged for you guys but yet I’m still VERY ecstatic! Wanna know why? Cuz it’s the last reflection!!! And I have been given the honor to do the last blog… kinda sad at the same time… 😦 cuz I will be the one to reminisce on the past! Don’t get me wrong though! I’m not complaining! It’s my privilege to reflect on the journey throughout this blog. Learning about blogs, expressing myself and sharing information for the world to read for the first time, made me a little nervous yet excited. It helped me try my best knowing that there are anonymous people out there from all corners of the globe that may stumble upon my work! This semester was a lot more hectic than the first yet it seemed to just fly-by. I assume it was due to me settling in being a freshman and all. In terms of Biochemistry, this course I personally consider very interactive and fun. It indoctrinated me into this mind-set of doing work, work, and work. All the credit goes to my hardworking, extremely friendly and down-to-Earth lecturer, Mr. Jason Matthew, AKA JM. This guy has given us podcast videos on almost all the topics. How awesome is that? I must say they were really helpful in making me understand the course and I was not bamboozled by most of the information even after watching the videos after the first time! Oh damn! It seems I am getting carried away here and forgetting the real purpose of this reflection! So down to the heart of the matter… this reflection is all about Nucleotides and Nucleic Acids!
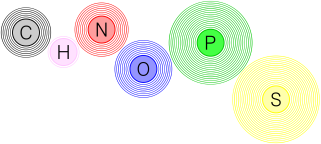
Nucleic acids are one of the four basic kinds of organic molecules made up of DNA and RNA they consist of all the CHNOPS elements excluding sulphur. This funny abbreviation (CHNOPS) stands for the elements: carbon, hydrogen, nitrogen, oxygen, phosphorus and as you could infer from above, the S stands for sulphur lol.
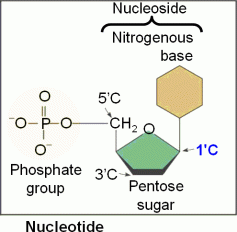
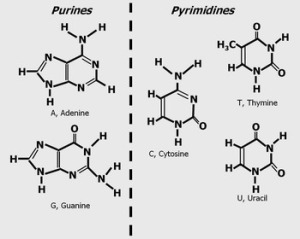
DNA stores genetic information and it is transferred from the nucleus to the ribosome via a type of RNA called messenger RNA (mRNA for short). I must say… such a small structure has a lot of information, it’s amazing how interesting our life can be! Nucleic acids are polymeric nucleotides that also make up proteins and also ATP an energy transfer agent. ATP is a nucleotide that provides energy for most cellular functions, it undergoes hydrolysis when there is a chemical energy change in the molecule where it loses a phosphate converting ATP to ADP. Nucleotides are the building blocks of DNA, RNA and nucleic acids. They are made up of phosphate groups essential for nucleotide polymerization (with a strong negative charge), pentose sugars (that in polymer biochemical structures creates a sugar backbone) and a nitrogenous base that differs in each nucleotide. The base sequence in DNA (which has a double helix) contains the following nucleosides A, C, G, T while in RNA strands, (a single helix), T is replaced by U. Nucleosides can be classified into 2 categories based on their size Purines with 2 rings (larger) and Pyrimidines with one ring (smaller). Purines are Adenine and Guanine while the pyrimidines are thymine, cytosine and uracil… in you guessed it… RNA lol
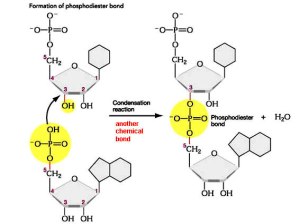
Two nucleotides are bonded by a phosphodiester linkage and a covalent bond is formed between the OH on the 3’ (read as 3 prime) nucleotide and the phosphate of the other.
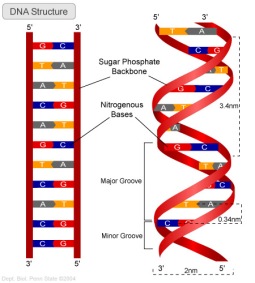
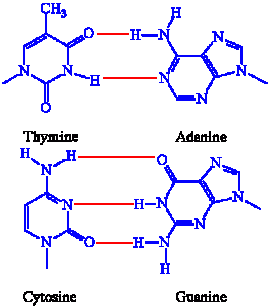
As mentioned before, DNA is a double helix. Its strands are antiparallel forming hydrogen bonds A to T and C to G while A to U and C to G in RNA. Antiparallel infers that one strand runs from the 3’ to 5’ end while the other is opposite. An illustration of this is provided bellow that would help you to visualize the principle:
Nucleotides bond in the 3’ and 5’ areas of their structures and this allows for the helical structure with the purine and pyrimidines bases on its inside and the sugars and phosphate on the outside of the DNA helix. There is antiparallel complementary base pairing where the hydrogen bonds hold the structures together.
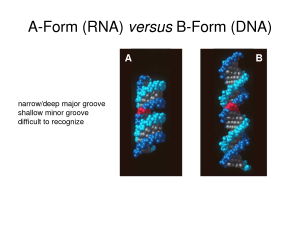
Nucleic acids have been said to be the major compounds of all life as Polynucleotides in the form of DNA and RNA are the basic structure that make up and synthesize everything alive. The nitrogenous bases attach to the C-1’ of the ribose or deoxyribose, while the pyrimidines bond at the N-1 on the pentose and Purines through the N-9 position. Nucleic acids are in three forms they are B form which is seen in DNA, A form which is familiar to RNA structures and Z form, a seldom observed structure seen in some DNA sequences. These structures are part of what allows for the stability of nucleic acids, the stacking interaction or hydrophobic interaction of the bases allows for the expulsion of water in the structure to aid in stability when they stack on each other. Nucleic acid can be affected by strong acids and high temperature since it hydrolyzes phosphate riboses and deoxyroboses. High pH may have little effect on DNA structure but may cause changes in the isomeric forms of bases affecting their connectivity; this tautomeric change results in DNA denaturation.
So! Back to my philosophy from the beginning of this blog! Now what was I talking about… oh right! I was talking about our insightful lecturer Mr. JM and his awesome vids! Well apart from enjoying my time at tutorials and lectures, I also enjoyed the conversations and ideas contemplated with my teammates during meetings for our blog! My time with my colleagues were really fun and productive. We all chipped in and helped each other in times of need when someone was stuck or in dire straits. There were areas where some of us were stronger and at times weaker. This is what helped each of us pull our weight and ensure that the blog was a success! 🙂 On behalf of the Biochemistry3rst team, Shiv Shiv (me lol), Rakeeru, Trav, Reshi and the Group Leader, Richie, an eleven week journey is never easy to conclude. Never starve your mind of knowledge because you afraid of an academic adventure. Biochemistry may buzz in your head, this may be painful at times, but the harder the battle, the sweeter the victory.
Biochemistry3rst over and out!
Reference:
Nelson, David, Michael Cox. 2005. “Nucleotides”. Lehninger Principles of Biochemistry, ed. Sara Tenney. New York: Freeman and Company.
Picture Reference:
- Reflecting…- http://langwitches.org/blog/wp-content/uploads/2011/06/experiences-reflection.jpg
- CHNOPS- http://thehypertextuallounge.files.wordpress.com/2013/09/chnops-svg.png
- Nucleotide- http://bio1151.nicerweb.com/Locked/media/ch05/05_26Nucleotide.gif
- Hydrogen bonds- http://biology.unm.edu/ccouncil/Biology_124/Images/DNAbases.gif
- Purines and Pyrimidines- http://biochemshariestar.files.wordpress.com/2013/04/image005.jpg
- Phosphodiester bond- http://assets.openstudy.com/updates/attachments/51efa293e4b00daf471a9a04-aaronq-1374994934903-phosphodiester.jpg
- Anti-parallel- https://wikispaces.psu.edu/download/attachments/46924774/image-1.jpg
- Goodbye- http://4.bp.blogspot.com/-MNhFkHfPlag/TeIor1jtkMI/AAAAAAAAALw/-cfCg8caf-c/s1600/good-bye.jpg
- Goodbye- Air Supply- http://www.youtube.com/watch?v=J6qxMP3deU8