REFLECTION 2- Carbohydrates
The Wonderful World of Carbohydrates!
Hello my fellow Biochemians!! This is Reshi here. This week in Biochemistry class, we talked about carbohydrates. I missed the first lecture on this topic, and I was a little upset about that. Thank goodness our lecturer was so thoughtful to make podcasts on these topics!
When our lecturer had mentioned that this topic had some Chemistry related areas, I started to panic. It is a known fact by all that I am no lover of Chemistry. However, after going through the slides in class, I realized that there was nothing to fear. Thankfully, I also have a chemistry background, which made it easier for me to understand the little technical pieces of the carbohydrate lecture. Anyway, enough about me and my phobia of chemistry. Let’s talk about Carbohydrates!
The first things that come to my mind when I think about carbohydrates are bread, rice and anything with sugar in it! But what exactly is a carbohydrate? This can be defined as a macromolecule that is made up of Carbon, Hydrogen and Oxygen atoms. Carbohydrates have a number of benefits to us humans, the most significant being as an energy source for the body. I have heard of people who have completely cut out carbohydrates from their diet in an attempt to lose weight. I really don’t think that is a very smart practice, because the brain and the Central Nervous System are solely dependent on glucose to function.
Carbs and our culture here in T&T
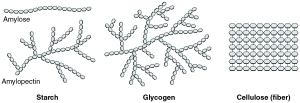
Carbohydrates are also used as energy storage. It is important to remember that starch is only found in plants, while glycogen is exclusive to animals. The structure of glycogen is more branched than that of amylopectin (a component of starch) because animals require rapid release of energy in certain situations. More branching allows for more surface area for enzymes to act upon and hence, a faster rate of energy release.
Cellulose and chitin are both carbohydrates that have structural roles. Cellulose is formed in plant cell walls and is a polymer of β-D- glucose. Chitin, on the other hand, is found in the exoskeleton of some insects.
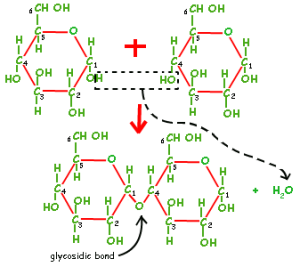
Now, carbohydrates can be either monosaccharides, disaccharides, oligosaccharides or polysaccharides. Monosaccharides (e.g. glucose, fructose) are simple sugars that can be either aldoses (having an aldehyde group at one end) or ketoses (having a keto group, usually located at Carbon 2). In aqueous solutions, the carbonyl group combines with an –OH group to form a cyclic compound (hemiacetal or hemiketal).
Note: A hemiacetal molecule can be formed when an aldehyde group and an alcohol group react. When a ketone functional group reacts with an alcohol group, a hemiketal structure is formed.
Before I go further, I wanted to point out something that had caught my attention in class. It is important to note that both glucose and fructose are hexose sugars. It may seem that fructose is a pentose sugar because it has a pentagonal shape, but this is a wrong assumption. The shape has nothing to do with it; it all depends on the number of carbons present in the structure. Our lecturer had pointed out this difference to us in class and, I admit, if he had not done so, I would have made the same mistake.
There is a very important process that takes place when two or more monosaccharides come together, and that is condensation reactions. In this process, a hydroxyl group from one monosaccharide bonds with a hydroxyl group from another monosaccharide to form a glycosidic bond and water as a byproduct. The formation of these glycosidic linkages is essential in disaccharide and polysaccharide structures.
Some popular disaccharide molecules include maltose (two α-D-glucose molecules bonded between Carbon 1 of one molecule and Carbon 4 of another), lactose (two aldohexoses: β-D-galactose and glucose), and sucrose (glucose and fructose). Of the three disaccharides mentioned, sucrose is the only non-reducing sugar.
Polysaccharides, as the name suggests, is made up of several monosaccharide molecules that are joined together by glycosidic linkages to form long chains of carbohydrates, which may or may not become branched. The three main examples of polysaccharides are starch, cellulose and glycogen. To illustrate the differences among them, I think it is best to do this in a table format:
|
FEATURE |
STARCH |
CELLULOSE |
GLYCOGEN |
|
|
Amylose |
Amylopectin |
|||
|
Sugar component |
α- glucose |
α- glucose |
β- glucose |
α- glucose |
|
Linkage |
α (1-4) |
α (1-4) and α (1-6) |
β (1-4) |
α (1-4) and α (1-6) |
|
Function |
Food storage (in plants) |
Food storage (in plants) |
Structural |
Food storage (in animals) |
|
Branching in structure |
none |
Branching occurs every 12-20 glycosyl residues |
none |
Branching occurs every 6-10 glycosyl residues; contains more branching than amylopectin |
Before I conclude, I thought I would share something else that caught my attention. Every time I switch on the television, I would see these advertisements regarding artificial sweeteners, such as Splenda and Truvia. I would always wonder to myself, how these products are able to mimic the taste of sugar, and if they are really a healthy alternative to using sugar. So I did some research and found that products regarded as “sugar-free” use artificial sweeteners to enhance the flavour of their products. However some artificial sweeteners are derived from natural sources, such as the stevia plant. There are indeed certain benefits to using these kinds of sweeteners, in that they have virtually no calories so they are widely used as a method of weight loss. Also, diabetics can use artificial sweeteners as an alternative because, unlike sugar, artificial sweeteners generally don’t raise blood sugar levels since they are not carbohydrates. There was once a concern that the sweetener Saccharin was causing bladder cancer amongst its users, but this was only the case if it was used in very high amounts. According to the National Cancer Institute, there is no solid proof that these artificial sweeteners are carcinogens. So, maybe artificial sweeteners aren’t so bad after all! But I think I’ll stick to my regular sugar.
Well I hear a cricket game calling my name, so until next time, this is Reshi signing out!
References:
http://www.mayoclinic.org/artificial-sweeteners/art-20046936
http://www.britannica.com/EBchecked/topic/94687/carbohydrate/72617/Sucrose-and-trehalose#toc72618
http:// http://www.theorganicdietitian.com/wp-content/uploads/2013/08/Sugar-Image.jpg
http:// http://www.elanaspantry.com/blog/wp-content/uploads/2007/03/splenda.jpg
http:// images.tutorvista.com/cms/images/38/glycosidic-linkage.png
http:// http://www.1zoom.net/Animals/wallpaper/170045/z208.3/
http:// cnx.org/content/m46008/latest/219_Three_Important_Polysaccharides-01.jpg
http:// leavingbio.net/nutrition%20and%20food_files/Nutrition%20and%20Food_files/image007.gif
http:// http://www.sepscience.com/images//Articles/Issues/0310/Issue2/Mcabe/fig-1-mcabe.jpg
http:// static.howstuffworks.com/gif/diabetes-and-carbohydrates-1.jpg
http:// http://www.youtube.com/watch?feature=player_embedded&v=nBorvZEnFnA