Reflection 5- Enzymes
Hey people of the biochemistry and science nation. I’m Shiv Shiv and I’m here to talk about what life is without me the enzyme…I’m just kiddin. So yea enzymes, what are they? Why do we need it? Who discovered this? So many questions to be asked I shall begin to answer them all for you.
I’ve learned that the scientist Louis Pasteur, came up with an idea that yeast converted sugar to ethanol during a fermentation reaction. Still it wasn’t until chemist Edward Buchner proved that it wasn’t the yeast itself that caused the fermentation but the extracts, which he called zymase. This was how enzymes were found to be independent of the cell. A few decades later Jeans Sumner singled out a single enzyme molecule. Just imagine the world of opportunity and discovery which opened for Biochemistry. He called it urease which is a protein and so are the majority of enzymes. Further advancement into knowledge and technology described the enzyme as a catalyst which means that they speed up a reaction giving the constant product required without being chemically changed by the process.
Substrate is the name given to molecules in the beginning of enzymatic reaction and upon enzyme activity they are converted to products. The number of substrate molecules converted to the product in an enzyme molecule per second is called Kcat (Benjamin et al., 1997). The method of decreasing the activation energy is to speed up the overall reaction rate to form products and acquire equilibrium at a hastened paste. Oh! Just in case you didn’t know, the energy required to complete a chemical reaction is called the activation energy. In the reaction, only the time taken to complete the process changes as everything else remains constant. Proteins and sometimes RNA make up the structure of an enzyme in globular form as a tertiary or quaternary structure. Each enzyme reacts specifically with a substrate because of its hydrophobic and hydrophilic characteristics. The active site is a indent or cleft in an enzyme where the substrate binds with it. The combined structure of the enzyme and the substrate form the enzyme-substrate-complex. These characteristics are like the makers of the active site they move according to the surroundings forming certain structures that can only accept specific substrates; this shows that active sites of enzymes are specific to certain substrates.
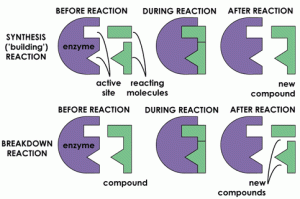
Specificity of enzymes is due to the shape and size of the active site. An enzyme may have the induced fit or lock and key method. The lock and key method was 1st postulated by Emil Fisher who I thank very much for having this part of biochemistry unlocked, as this indicated the specificity of the enzyme because the theory suggested that the enzyme has a certain shape that only some or a specific substrate may enter to react with the enzyme. The induced theory however, stated that an enzyme can stretch and be flexible to facilitate a substrate as long as it is compatible with the enzyme. Enzyme react in catabolic and anabolic phases. Catabolic reactions break down and release energy to drive a chemical reaction, this same energy is used in anabolic reactions to build , make new molecules and store energy.I got a wonderful illustration below to show to you!
Thousands of enzymes within the body are essential for an organism to survive and function properly. Some enzymes may not function without the aid of a nonprotein chemical component. The compound is known as a cofactor. Okay let’s not forget enzymes and inhibition, there irreversible inhibition and reversible inhibition. They can be told apart by the individual attraction for an enzyme. An irreversible inhibitor is formed where the bonds from the enzyme are “tougher than concrete”, making it hard to break apart permanently removing catalytic activity. This type of inhibitor is also called inactivators and can bond covalently to amino acids during catalysis in the active site. Allosteric site is a site on the enzyme that would allow another molecule to bind with it. The enzyme becomes unfit for substrates to bond with it. Inhibition can also be reversible, where the inhibitor forms a weak bond with the enzyme so it is easier to break apart to allow for a substrate to come back. There are four types of reversible inhibitions; they are: competitive, uncompetitive inhibition, mixed inhibition and non competitive inhibition. Competitive inhibition is where an inhibitor competes with a substrate to bond with an enzyme to inhibit its catalytic function. Non-competitive inhibition is where an inhibitor bonds permanently with the enzyme blocking all its enzymatic functions. The inhibitors main function is to decrease the enzymes ability to work on a substrate and lower the catalytic ability.
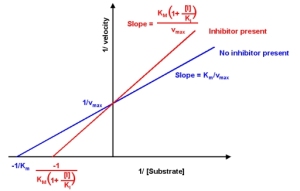
Lets focus a bit on competitive inhibition which can be illustrated in the Line-Weaver Burk plot below. In enzymology, when initial velocity of a reaction is plotted against substrate concentration, it is sometimes not possible to determine Vmax immediately. By reciprocating both values, the resulting Lineweaver Burk plot can be used as a graphical apparatus to observe mechanisms of inhibition.
The plot suggests that the effect of the inhibitor can be reversed by increasing substrate concentration since Vmax is constant. Furthermore, Km was seen to have increased(with inhibitor concentration) which implies that more substrate is required to achieve 1/2 Vmax (Substrate affinity decrease with the inhibitor).
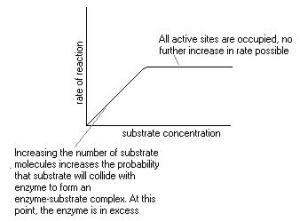
The concentration of a substrate and enzyme can affect the rate of reaction, by increasing its concentration present in the reaction, it will cause an increase in the reaction. If at one point the substrate remains constant and the concentration of enzymes increase then the rate increases linearly. When substrate concentration increases the rate of reaction will only increase for a certain period where it levels out and remains constant. At this point the enzymes can no longer increase in catalytic function and a line parallel to the x axis on the graph is formed, this is what is known as linearly increasing. Two other factors that affect the rate of reaction are the temperature and effect of pH. These can only positively affect the rate at their optimum levels. Any higher and denaturation begins, denaturation is the loss of catalytic function in an enzyme .Heat above 40 degrees Celsius along with strong acids or bases and inorganic salts in high concentration causes denaturation of enzymes.
Enzymes are used in digestion of food and metabolic enzymes. Food enzymes are found in… you guessed it food. It is also used for digestion in the digestive system and metabolic throughout the body. From food we can trace backward to how food is made and it is mostly from plants. Enzymes have been modified and used as fertilizers, pest control and additives for improvement of digestion in livestock. This is amazing for me to know that enzymes are so essential in the way man altered it to be used for protection of resources. The soil, water and quality of the goods produced are extremely upgraded after treatment of enzymes. Dry barren soil can be turned into rich vegetative soil just as plants can bear faster and bigger than without enzyme treatment to it. Interesting huh? Well I hope this was informative and that something that I talked about remains with you after reading this blog entry! Now it’s time for me to go… Look forward to Richie’s reflection on GLYCOLYSIS!!! Shiv Shiv over and out!!!
References:
- maltingandbrewing.com
- ww.benchfly.com
- http://www.seattleorganicrestaurants.com
- http://projectsharetexas.org/resource/role-enzymes?field_resource_keywords_tid=&sort_by=title&sort_order=ASC&items_per_page=50&qt-view__resource_content__panel_pane_1=2
- http://www.mansfield.ohio-state.edu/~sabedon/biol1045.htm
- http://www.slideshare.net/taymuimui/4-the-role-of-enzyme-in-agriculture
- http://www.chemistryexplained.com/Hy-Kr/Inhibitors.html
- science.howstuffworks.com
- education.yahoo.com
- www.elmhust.edu
- hartnell.edu
- barleymagic.com
Ramersar, Myda, Mary Jones, Geoff Jones. 2011. Biology for CAPE.
Picture References:
Louis Pasteur- http://images.fineartamerica.com/images-medium-large/1-louis-pasteur-1822-1895-french-chemist-everett.jpg
Activation energy- http://www.uic.edu/classes/bios/bios100/lectures/energyhump01.jpg
Enzyme Activity process- http://xn--82cyjg4au6jsb6c4bzc.com/image/data/enzyme-duty.gif
substrate concentration- http://physicsmadeeasy.files.wordpress.com/2007/11/substrateconc.jpg.
effect of temperature on enzyme
http:// http://www.bbc.co.uk/schools/gcsebitesize/science/images/add_edex_bio_temp-chart.jpg
enzyme ph- http://www.girton.vic.edu.au/biology/images/enzyme5.gif
keep calm- http://sd.keepcalm-o-matic.co.uk/i/keep-calm-and-stay-tuned-15.png.
You complete me- http://25.media.tumblr.com/tumblr_m9qu44ub541rcuee4o1_500.png
REFLECTION 2- Carbohydrates
The Wonderful World of Carbohydrates!
Hello my fellow Biochemians!! This is Reshi here. This week in Biochemistry class, we talked about carbohydrates. I missed the first lecture on this topic, and I was a little upset about that. Thank goodness our lecturer was so thoughtful to make podcasts on these topics!
When our lecturer had mentioned that this topic had some Chemistry related areas, I started to panic. It is a known fact by all that I am no lover of Chemistry. However, after going through the slides in class, I realized that there was nothing to fear. Thankfully, I also have a chemistry background, which made it easier for me to understand the little technical pieces of the carbohydrate lecture. Anyway, enough about me and my phobia of chemistry. Let’s talk about Carbohydrates!
The first things that come to my mind when I think about carbohydrates are bread, rice and anything with sugar in it! But what exactly is a carbohydrate? This can be defined as a macromolecule that is made up of Carbon, Hydrogen and Oxygen atoms. Carbohydrates have a number of benefits to us humans, the most significant being as an energy source for the body. I have heard of people who have completely cut out carbohydrates from their diet in an attempt to lose weight. I really don’t think that is a very smart practice, because the brain and the Central Nervous System are solely dependent on glucose to function.
Carbs and our culture here in T&T
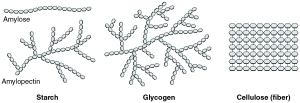
Carbohydrates are also used as energy storage. It is important to remember that starch is only found in plants, while glycogen is exclusive to animals. The structure of glycogen is more branched than that of amylopectin (a component of starch) because animals require rapid release of energy in certain situations. More branching allows for more surface area for enzymes to act upon and hence, a faster rate of energy release.
Cellulose and chitin are both carbohydrates that have structural roles. Cellulose is formed in plant cell walls and is a polymer of β-D- glucose. Chitin, on the other hand, is found in the exoskeleton of some insects.
Now, carbohydrates can be either monosaccharides, disaccharides, oligosaccharides or polysaccharides. Monosaccharides (e.g. glucose, fructose) are simple sugars that can be either aldoses (having an aldehyde group at one end) or ketoses (having a keto group, usually located at Carbon 2). In aqueous solutions, the carbonyl group combines with an –OH group to form a cyclic compound (hemiacetal or hemiketal).
Note: A hemiacetal molecule can be formed when an aldehyde group and an alcohol group react. When a ketone functional group reacts with an alcohol group, a hemiketal structure is formed.
Before I go further, I wanted to point out something that had caught my attention in class. It is important to note that both glucose and fructose are hexose sugars. It may seem that fructose is a pentose sugar because it has a pentagonal shape, but this is a wrong assumption. The shape has nothing to do with it; it all depends on the number of carbons present in the structure. Our lecturer had pointed out this difference to us in class and, I admit, if he had not done so, I would have made the same mistake.
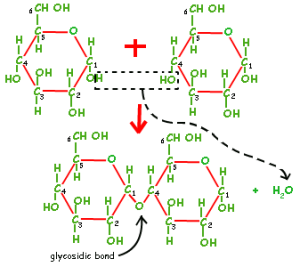
There is a very important process that takes place when two or more monosaccharides come together, and that is condensation reactions. In this process, a hydroxyl group from one monosaccharide bonds with a hydroxyl group from another monosaccharide to form a glycosidic bond and water as a byproduct. The formation of these glycosidic linkages is essential in disaccharide and polysaccharide structures.
Some popular disaccharide molecules include maltose (two α-D-glucose molecules bonded between Carbon 1 of one molecule and Carbon 4 of another), lactose (two aldohexoses: β-D-galactose and glucose), and sucrose (glucose and fructose). Of the three disaccharides mentioned, sucrose is the only non-reducing sugar.
Polysaccharides, as the name suggests, is made up of several monosaccharide molecules that are joined together by glycosidic linkages to form long chains of carbohydrates, which may or may not become branched. The three main examples of polysaccharides are starch, cellulose and glycogen. To illustrate the differences among them, I think it is best to do this in a table format:
|
FEATURE |
STARCH |
CELLULOSE |
GLYCOGEN |
|
|
Amylose |
Amylopectin |
|||
|
Sugar component |
α- glucose |
α- glucose |
β- glucose |
α- glucose |
|
Linkage |
α (1-4) |
α (1-4) and α (1-6) |
β (1-4) |
α (1-4) and α (1-6) |
|
Function |
Food storage (in plants) |
Food storage (in plants) |
Structural |
Food storage (in animals) |
|
Branching in structure |
none |
Branching occurs every 12-20 glycosyl residues |
none |
Branching occurs every 6-10 glycosyl residues; contains more branching than amylopectin |
Before I conclude, I thought I would share something else that caught my attention. Every time I switch on the television, I would see these advertisements regarding artificial sweeteners, such as Splenda and Truvia. I would always wonder to myself, how these products are able to mimic the taste of sugar, and if they are really a healthy alternative to using sugar. So I did some research and found that products regarded as “sugar-free” use artificial sweeteners to enhance the flavour of their products. However some artificial sweeteners are derived from natural sources, such as the stevia plant. There are indeed certain benefits to using these kinds of sweeteners, in that they have virtually no calories so they are widely used as a method of weight loss. Also, diabetics can use artificial sweeteners as an alternative because, unlike sugar, artificial sweeteners generally don’t raise blood sugar levels since they are not carbohydrates. There was once a concern that the sweetener Saccharin was causing bladder cancer amongst its users, but this was only the case if it was used in very high amounts. According to the National Cancer Institute, there is no solid proof that these artificial sweeteners are carcinogens. So, maybe artificial sweeteners aren’t so bad after all! But I think I’ll stick to my regular sugar.
Well I hear a cricket game calling my name, so until next time, this is Reshi signing out!
References:
http://www.mayoclinic.org/artificial-sweeteners/art-20046936
http://www.britannica.com/EBchecked/topic/94687/carbohydrate/72617/Sucrose-and-trehalose#toc72618
http:// http://www.theorganicdietitian.com/wp-content/uploads/2013/08/Sugar-Image.jpg
http:// http://www.elanaspantry.com/blog/wp-content/uploads/2007/03/splenda.jpg
http:// images.tutorvista.com/cms/images/38/glycosidic-linkage.png
http:// http://www.1zoom.net/Animals/wallpaper/170045/z208.3/
http:// cnx.org/content/m46008/latest/219_Three_Important_Polysaccharides-01.jpg
http:// leavingbio.net/nutrition%20and%20food_files/Nutrition%20and%20Food_files/image007.gif
http:// http://www.sepscience.com/images//Articles/Issues/0310/Issue2/Mcabe/fig-1-mcabe.jpg
http:// static.howstuffworks.com/gif/diabetes-and-carbohydrates-1.jpg
http:// http://www.youtube.com/watch?feature=player_embedded&v=nBorvZEnFnA