REFLECTION 9- LOVELY LIPIDS PART 2
Hey there folks!! Rakeeru here and it’s finally my turn again to share some thoughts on this blog. This week was pretty much wacky because there was so much to do and so little time to do it. I kid, I kid! I ‘m a master procrastinator but nothing can stop me from sharing some cool stuff about lipids. My love for lipids will never die… lol. My mission is to help you understand lipids in a fun and enjoyable way. So we are going to be looking at three types of structural membranes of lipids, phosphodiate, Sphingosine and cholesterol.
Let’s talk GLYCEROLPHOSPHOLIPIDS! So you guys should be familiar with the structure of glycerol, but if you aren’t, then let’s recap a bit. Glycerol is an alcohol that has three carbon atoms, each bonded to an alcohol group. Okay let’s go back to the glycerol phospholipids now. A Glycerol phospholipid, also known as a phosphodiate, has a glycerol as his backbone, where on carbon 1 only a saturated fatty acid chain is attached. On carbon 2 an unsaturated fatty acid chain is bonded and last but not least on carbon 3 a phosphate group is directly bonded to it. Note that when an alcohol is attached to an acid group it forms an ester bond. Look at the lovely diagram below.
Now this entire molecule is said to be amphipathic, meaning it has a polar end and a non-polar end. (See picture below). The polar end is hydrophilic or “water loving” while the non-polar end is hydrophobic or “water hating”. The polar end is the made up of a phosphate group and a hydroxyl group.
(R1 and R2 represent the fatty acid chain , the X represents the hydroxyl group) So Trav told you about the ability of unsaturated fatty acids to form a kink, and I just want to add a few things to that… Not all unsaturated fatty acids show a kink (changes direction of the chain). Unsaturated fatty acids can either be in cis configuration or trans configuration. ONLY CIS fatty acid chains display that appearance due to the fact that the C-3 and C-6 are on the same side of the double bond. (see the diagram below). In a trans fatty acid chain, the structure does not show any kinks. Instead, it follows the pattern of the fatty acid. It resembles the saturated fatty acid so I call it “the sly one”, since I cannot tell which one is the unsaturated fat and which in the saturated. O Boy, knowing that all the good-tasting stuff (junk food) is high in trans-fat which is terrible for health is a little scary, since the transfatty acid is packed tightly which increases the LDL( Low Density lipoprotein) and decreases HDL (High Density lipoprotein).
Okay moving on.. moving on… Another amazing lipid is… SPHINGOLIPID!!! 😀 This structure has a sphingosine backbone; sphingosine is an alcohol that has 18 CARBONS. There are many different functional groups attached to it. On carbon 1 and 3 there are hydroxyl groups present, an amino group attached to carbon 2 and a trans double bond is present in the middle of carbon 4 and 5. (See drawing below)
Remember I mentioned sphingosine is the backbone of the sphingolipid. What makes it a sphingolipid? It’s the presence of a saturated fatty acid chain. This fatty acid attaches to the carbon 2 that is bonded to an amino group to form an amide bond.
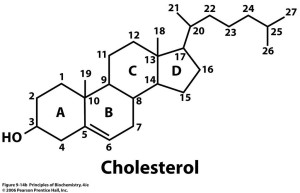
On carbon 1, there is an R group; this is simply any other group that is attached to the carbon, for example if hydrogen is R group then the name of the entire structure is ceramide. Ceramide is an oily substance that is found on the outer layer of the skin. It basically adds moisture to skin, which helps to prevent skin from drying and flaking. (Curel, 2014). Another example is sphingo myelin, which is created when the R group is phospocholine. The sphingo myelin is found in red blood cells and myelin sheaths. What captured my attention is that sphingo myelin increases the speed at which nervous impulses conduct themselves by insulating the nerve fibers. (Voet, Voet and Pratt, 2008). Pretty kool stuff. 😀 Last but not the least, Cholesterol!!! I’m sure you heard of this word before. But what exactly is cholesterol? Well here goes, cholesterol is a molecule that has 27 carbons. This molecule has 3 rings that have 6 carbon atoms and 1 ring that only has 5. These rings are referred to as steroids and are stuck together. Looking at ring B there is a double bond between carbon 5 and 6. There is a hydroxyl group on the carbon 3 atom and there is an alkyl group on the carbon 17. There are 2 methyl groups, one on carbon 19 and the other on carbon 18. By the way, in case you forgot what an alkyl group is, it’s a fragment of a molecule that has a general formula (CnH2n+1,). (Senese, 2010).
Well what’s so important about cholesterol? Believe it or not, without cholesterol we wouldn’t even exist. You know what’s amazing? The body is able to produce cholesterol on its own without consumption of food rich in cholesterol. (Mason W. Freeman, 2014). It plays an important role in the body:
- it forms bile acids to make digestion a lot easier in the intestine,
- Having cholesterol allows the body to produce vitamin D or hormones like testosterone. Testosterone is a steroid hormone that is produced in large amounts in males. It is responsible for the defining characteristics in men, and also for overall health and wellbeing.
- Cholesterol, together with phospholipids, makes up the membrane of cells in animals. Each cholesterol molecule attaches itself to a phospholipid group. The hydrophilic end of the cholesterol (where the hydroxyl group is located) is close to the hydrophilic end of the phospholipid. The hydrophobic portion of the cholesterol interacts with the hydrophobic portion of the phospholipid.
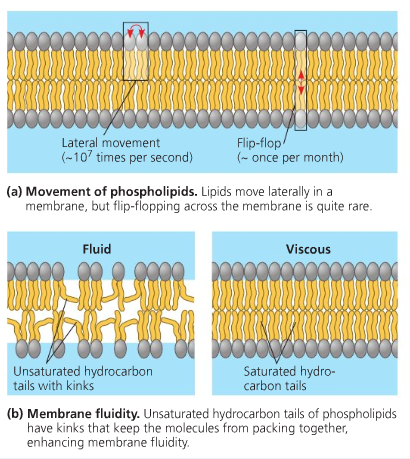
Just to spice things up a bit, have you ever wondered how cholesterol affects the fluidity of the membrane? Well it all depends on the temperature. In high temperature the cholesterol controls the movement of the phospholipid and lessens the fluidity. Heat energy encourages the phospholipids to move a lot which will destabilize the membrane. In low temperature the cholesterol controls the phospholipid by avoiding compaction. In cold conditions the phospholipid moves less and will become rigid.
Well that’s it for now! I hope my reflection was very informative and you guys learnt from this… Biochemistry is fun! BEST OF LUCK IN YOUR STUDIES!!! 😦 Unfortunately this is my last reflection… but my best buddy Shiv Shiv will do a tremendous job! Next up is nucleotides.. soo stay tuned… Until next time Rakeeru out!
References:
- Curel. 2014. <https://www.curel.com/experts-corner/ceramides.aspx>.
- Mason W. Freeman, M.D. with Christine Junge. Harvard University . 2000-2014. <http://www.health.harvard.edu/newsweek/Understanding_Cholesterol.htm>.
- Senese, Fred. General Chemistry online. 1997-2010. <http://antoine.frostburg.edu/chem/senese/101/organic/faq/yl-ending.shtml>.
- Voet, Donald J., Judith G. Voet and Charlotte W. Pratt. ” Bilayers and Membranes.” Principles of Biochemistry third edition. 2008. 252.
Picture References:
- Gylcerolphospholipid-http://classconnection.s3.amazonaws.com/365/flashcards/272365/png/glycerophospholipid_general_structure1335420825845.png
- Polar and non-polar of phospholipid-http://upload.wikimedia.org/wikipedia/commons/thumb/f/fd/Phospholipid.svg/290px-Phospholipid.svg.png
- cis and trans fatty acids-http://www.mansfield.ohio-state.edu/~sabedon/047cis.gif
- sphingosine- https://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb1/part2/images/sphing.gif
- sphingosine fatty acid-http://upload.wikimedia.org/wikipedia/commons/a/a7/Sphingolipid.png
- cholestorol-http://photos1.blogger.com/blogger2/6245/4534/1600/figure%209-14b.jpg
- Procrastination- http://www.quickmeme.com/img/95/95b5d62601a8d7a4b79ff15c5da165827719f3defa11fd02b5be11b6d6534de3.jpg
- Paula and Butter- https://i.chzbgr.com/maxW500/5591723264/h02E9A76E/
- Lipids are good- http://memecrunch.com/meme/2BR64/lipids/image.png
- Fluidity of membrane- http://web.sls.hw.ac.uk/teaching/Derek_J/A13MM1-web/files/revision_stuff/membranes/files/3_12.jpg
Reflection 8 –Lipids 1
Hey y’all! This is your boi Trav here again bringing to you yet another reflection! This one being on… drum roll please… Lipids! Yes Lipids! How fortunate am I to do another topic that interest me due to my current lifestyle… you know with the gym and all… Well what can I say about lipids… when I think of lipids and I think about Fats… when I think about fat, I think about losing it! Lol. Yes, yes I know fats aren’t all bad. So! I’m sure you guys are dying to know what lipids are, so let me get started. 🙂
Okay, so Lipids are made up of long chains of carboxylic acids that are the building blocks of most lipids. It is made up of a wide range of different classes. These common classes of lipids include: Fatty Acids, Triacylglycerol, Phospholipid, Glycerol, and Steroid. For this reflection, you would be seeing three words a lot. They are: Fats, Oils and Lipids. Lipids are used to refer to both fats and oils, Fats are used to refer to the solid form of lipids and well you can guess what oils are used to refer to. Yes, it’s when we talk about the liquid form!!! Fats are solids because they are made up of carbon chains saturated with hydrogen atoms, while oils contains unsaturated hydrocarbon chains. Oils can have at least one carbon-carbon double bond that disrupts complete saturation and if there are more than one, it is termed polyunsaturated. Oh, I should emphasize that since fats are saturated hydrocarbons, they do not have any carbon-carbon double bonds. There is also a substance known as Wax which is made up of a Fatty acid and an alcohol (long chain). Waxes are important in fruits for protection and also appearance. Fatty acids comes in different chain lengths: Short (less than 6 carbons), Medium (6-12 carbons) and Long (more than 14 carbons).
Fatty acids have some rather important functions for organisms that include:
- Insulation from the cold (bears during hibernation),
- Energy source/storage,
- Helps cushion and protect your vital organs such as your kidneys, liver and heart.
Fat will give you more energy per gram than carbohydrates since it is in a more reduced form. This reduced form indicates that there is a lot less oxygen than a carbohydrate which means a lot more oxidation would take place. More oxidation means more energy. They supply Essential Fatty Acids such as Linoleic and linolenic acids. Fatty acids also help form the cell membrane of cells due to the phospholipids bilayer.
Saturated Fats are all bonded to Hydrogen and as stated before there are no C-C double bonds. They have long, straight chains and it is solid at room temperature. Unsaturated fats are those with the presence of the carbon-carbon double bonds. Unsaturated fats are liquid at room temperature because in the cis form, kinks where double bonds are found, prevents molecules from being tightly packed hence they are in the liquid form.
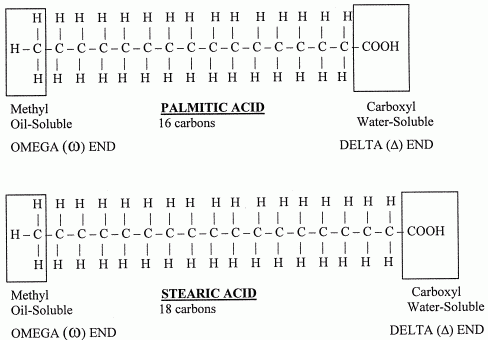
So what is the structure of Fatty acids? Fatty acids are made up of 2 different ends, the methyl and carboxyl end and many Hydrocarbon bonds (CH2) in between the 2 ends. A wonderful diagram is provided for you below :).
What caught my interest is the patterns found in fatty acids. One being that there is an even number of carbon atoms. The other is the location of the double bonds… for most monosaturated fatty acids (1 double bond), the double bond is found between carbons 9 and 10 while for polyunsaturated fatty acids, it is usually found at carbons 12 and 15.
Now I shall talk about the characteristics and trends that occur in fatty acids. As the number of Carbons in the Fatty Acid Chain increases, the Melting point increases. Also when the number of carbons increases, the Solubility in water decreases. An interesting point to note is that the melting point varies for saturated fatty acids and for unsaturated fatty acids. For example, between Stearic acid and Linoleic acid… Stearic Acid is a saturated fatty acid which means there are no double bonds. These are solid and also are tightly packed molecules. Linoleic acid is an unsaturated fatty acid and contains 2 double bonds. If you remember from what I mentioned above, unsaturated fatty acids are liquid at room temperature and they are not tightly packed. These characteristics proves that Linoleic Acid has a lower melting point than Stearic acid, having a melting point of -5°C and stearic acid having a melting point of 69.6°C. Oh! Something to note… as the number of double bonds increases the melting points decreases.
Lipids also have a physiological function: It is said that the chain length and amount of fatty acids is important for the fluidity of the membrane. It needs to be regulated in order to maintain this fluidity of the membrane lipids. Unsaturated Fatty acids especially are important as it promotes the fluidity. This is due to the unsaturated fats’ kink which creates something called the “ elbow room” that forms a space in between fatty acids giving enough room to ensure that the membrane is not too rigid.( Cnx Biology, 2013.)
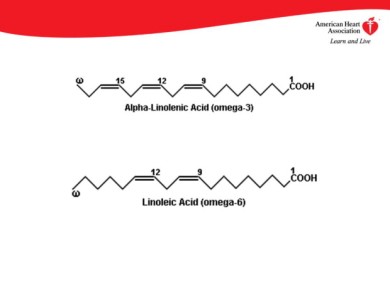
Essential Fatty Acids are those Fatty Acids that cannot be made by the human body. So how is it that we acquire these essential fatty acids? Well just like for amino acids, they are gained by the food that we consume and digest. Good examples of Essential Fatty acids are the omega-6 linoleic acid and omega-3 alpha-linolenic acid. They are rather important for human health. I will discuss the benefits of these a little later, so something to look forward to! So what do you think Non-essential Fatty acids are and where do you think we attain these? Well I am sure you guessed that these are produced by the body and therefore not needed in our diet. Oh and like I stated for amino acids, essential and non-essential substances are both important, not just the essential fatty acids. Lol Déjà vu moment…
Now something a little different, what we are gonna talk about is what happens when we add hydrogen to an unsaturated fatty acid. What happens is, first the carbon-carbon double bond is broken. This leaves room for the carbon to bond to another hydrogen. This produces only single bonds and this process is known as Hydrogenation. Better manipulation and shelf life increases for the food industry by this process. This process also produce the “bad” fats known as Trans Fats and they are found in most fried foods as well as margarines. Trans fat is created when hydrogen is added which makes the oil last longer. The problem with it is that it increases your Low-density Lipoprotein (LDL) Cholesterol and decreases your High-density Lipoprotein (HDL) Cholesterol. LDL Cholesterol leads to a build-up in the artery which can lead to atherosclerosis. This means that it increases one’s risk of getting a heart attack. Yikes! That’s not good! 😦
That was a little scary so now something to make the mood lighter! So, have you guys wondered how you can measure and test whether a substance is saturated or not and if it is unsaturated to what degree? Umm I will go ahead and assume no… lol. Okay… so yep, there is a way by introducing iodine and measuring the amount of iodine that can react with the unsaturated fatty acid. This is called the Iodine Index. The higher the percentage of unsaturated fats found in the food/ substance, the higher the Iodine index will be. It is similar to hydrogenation as it breaks the double bond and bonds to the molecule. Oh and it is important to note that 1 mole of Iodine reacts with the compound.
Glycerol! Yay! Time to talk about it lol. These guys got three hydroxyl groups and three carbons attached to them! What is special about glycerol is that they can combine with three fatty acids on the hydroxyl group. This is possible because the hydroxyl group on the glycerol combines with a carboxyl group of the fatty acid. This undergoes a condensation reaction where a water molecule is lost and the bond formed is known as an ester bond. The compound that is produced from this reaction is known as a Triacylglycerol or TAG molecule. One thing to note is that the TAG can also be hydrolysed using an enzyme known as lipase to break it down back into the Glycerol and the three fatty acids. It is also possible to break down a triglyceride using a strong base to convert it into a soap and a glycerol molecule.
Well that’s it for Lipids! Don’t go yet though! Just as I stated earlier, I got some information on the benefits of omega-6 linoleic acid and omega-3 alpha-linolenic acid to share with you! These two acids are Polyunsaturated Fatty acids (PUFA). They are also essential fatty acids as they cannot be produced by the body and are therefore acquired from your diet. For omega-3, food sources include: Canola oil, Flax seeds, Seafood, Pumpkin seeds, Eggs and many more… For omega-6 sources include: Soybean oil, corn oil, sunflower oil, canola oil and eggs again. I know you guys must be fed up of reading so I will put the benefits of these acids in point form.
- Helps lower the “bad” LDL cholesterol which ensures the decrease in cardio vascular disease potential.
- Helps reduce inflammation which prevents many different types of illnesses
- Helps with growth and development
- Important for brain development
Okay… it seems I am all out of juice… no more information for me to share with you… sniff sniff :(… wish I could have continued the rest of Lipids but that’s the job for our next reflector… my good friend Rakeeru! So see you guys around… Hope this wasn’t too tedious and long to read. Hope you guys learnt something new from all this. So! Until next time Trav out!
References:
http://cnx.org/content/m44416/latest/#fig-ch05_01_03
http://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/in-depth/trans-fat/art-20046114
http://www.webmd.com/cholesterol-management/ldl-cholesterol-the-bad-cholesterol
Picture References:
- Fatty acids, Steroids, Phospholipids, Triglyceride-http://cnx.org/content/m47834/latest/Figure_02_03_06.jpg
- Essential fatty acids- http://www.bodybuilding.com/fun/images/2007/md96.jpg
- Fatty acid Structure- http://www.intechopen.com/source/html/42113/media/image3.png
- melting point of lipids- http://180.211.114.59:1111/econtent/lipids-I/fatty-acids.php
- fluidity- http://test.classconnection.s3.amazonaws.com/794/flashcards/406794/png/fluidity.png
- artherosclerosis- http://www.yohyoh.com/health/images/diseases/atherosclerosis/atherosclerosis-and-dite.jpg
- iodine index of lipids- http://www.emeraldinsight.com/content_images/fig/0180500102003.png
- glycerol- http://hyperphysics.phy-astr.gsu.edu/hbase/organic/imgorg/glycerol.gif
- triacylglycerol- http://www.galilu.com/chem/triacyl.gif
- Linolenic & Linoleic Acid- http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/image/ucm_433120@z_extracted~1/large.jpg
- Out of juice- http://www.geekdip.com/wp-content/uploads/2012/10/MaxMemeJuice.jpg
- Structure of a fatty acid-http://www.benbest.com/health/efastru2.gif